We’ve already seen how to build electron configurations
Its time to talk about the Quantum numbers
Quatum numbers of the LAST electron of Sulfur
First you will have to find out where is located the electron, the last electron is here
Draw squares, one for each type of orbital, for p:
Now find the middle square and mark it as 0, squares in the right are positive, squares in the left are negative.
How many electrons where on this subshell (for Sulfur)? There were 4 so we only fill 4 electrons; the last one you marked is the last electron.
Upward arrows have S = ½ and downward arrows have S = - ½
n = 3
m = -1
s = -1/2
Now lets get the Quantum numbers for the Sulfur 9th electron
n = 2
m = 0
s = -1/2
Electron Configuration for Excited State
In ground state the number of electrons is equal to the number of protons giving the atom a neutral charge.
In excited charge status the atom has lost/gain electrons, this can be because of a chemical reaction or radiation.
Sodium (Na) has 11 electrons, but what happens with excited Sodium (Na+1)
As you can see the charge of Sodium is +1, that indicates that the atom has lost 1 negative charge being that an electron; so excited sodium has 10 electrons, 1 less than its ground state.
Oxygen (O) in ground state has 8 electrons, but excited Oxygen (O-2) has a negative charge of -2, meaning that it gained 2 electrons, getting 10 total electrons on excited state.
The process of building the electron configuration now is the same, just make sure you have the correct number of electrons for that atom.
Ground State
O = 1s2 2s2 2p4
Excited State
O-2 = 1s2 2s2 2p6
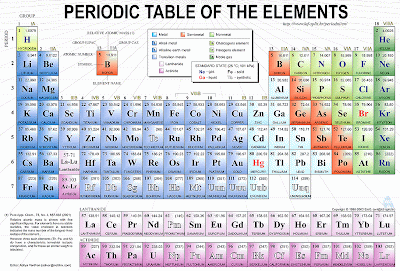
Some Periodic tables come with electron configurations included, but in that tiny square the whole configuration of lets say, Lead that has 82 electrons, wouldn’t fit in, that’s why we use one of our friends, the noble gases.
A peculiar characteristic of these elements is that the last shell of the atom is ALWAYS FULL.
So we use them to avoid writing a long electron configuration:
Lead, has 82 electrons:
Pb = 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2
Pretty long right? Now lets make the configuration of the Noble gas BEFORE Lead.
Xenon: with 54 electrons:
Xe =1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
The short configuration for Lead would be:
Pb = [Xe] 6s2 4f14 5d10 6p2
This is what you will find in some Periodic Tables.
Coming up!! Inorganic Chemical Reactions: Oxides