The periodic table of elements classifies, organizes and distributes the chemical elements, according to their properties and characteristics.
The creation of the periodic table can be attributed to Dmitri Mendeleyev, although wasn’t the only one, there were more scientists researching and trying to organize all the chemical elements discovered at their time.
 |
| Source: Wikipedia.org |
We have discovered 118 elements so far. At first elements are arranged by atomic number, Atomic number (Z) represents the number of PROTONS in the nucleus, not to be confused with Mass Number.
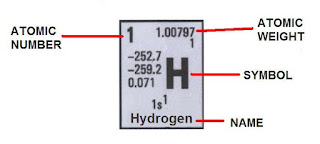
This can be found (In most tables) here:
As you can see element’s atomic number increases as you go RIGHT and DOWN.
 |
| Source: Modified table from http://www.periodni.com by Aditya Vardhan |
Horizontal Rows are called PERIODS and Vertical Columns are called GROUPS; elements on the same group have similar configurations of the outermost electron shells of their atoms (on how many electrons are on that last shell), and elements on the same Period have the same number of electron shells.
There are 18 groups and 7 periods.
Basic periodic tables give at least the abbreviation, the atomic number and mass weight.
More advanced tables give more information, like the oxidation state, electronegativity, the boiling and melting point, electronic configuration, density and more.
Mass number (A) is the sum of the number of protons (atomic number Z) and neutrons (N), and the number of electrons is the same as protons giving a neutral charge (on a primary state).
So basically:
Mass # = Atomic # + # Neutrons
A = Z + N
ISOTOPES
In the nature there is more than 1 kind of atom of the same element, they are called ISOTOPES. The only difference between isotopes is the number of NEUTRONS, therefore different Atomic Number; they have the same number of protons and electrons though.
To give an example lets see the isotopes of Carbon (C),
- Carbon-12, the most common accounting for 98.89% of total carbon in the nature.
Mass Number (A) = 12
Atomic Number (Z) = 6 --> every element including isotopes have the
same atomic number.
Number of Neutrons (N) = A – Z = 12 – 6 = 6
- Carbon-13, that makes up about 1.1% of all natural carbon on Earth.
Mass Number (A) = 13
Atomic Number (Z) = 6
Number of Neutrons (N) = A – Z = 13 – 6 = 7
- Carbon-14, this one only occurs in trace amounts, something like 0.000001%
Mass Number (A) = 14
Atomic Number (Z) = 6
Number of Neutrons (N) = A – Z = 14 – 6 = 8
Don’t confuse mass number (A) with ATOMIC WEIGHT; mass number of an atom as we saw is the sum of neutrons and protons; in the other hand Atomic Weight is the average atomic mass of the different isotopes of that element (weighted by abundance)
To avoid trying to understand a difficult equation lets see examples:
Carbon, 3 isotopes C-12 C-13 C-14
Note: Whenever you may need the atomic weight, use ONLY the rounded number in the periodic table, e.g. Carbon = 12.0112 but we use 12, Oxygen weights 15.9994 but we use 16.
Coming up Next! Getting the most of your Periodic Table: Electronic Configurations
For those in need: Ger your own Printable Periodic Table
Want to learn More?: How to use your Periodic Table (Advanced)
For those in need: Ger your own Printable Periodic Table
Want to learn More?: How to use your Periodic Table (Advanced)









7 comments:
Periodic table brings back the good old school days :).
This always confused me when I was at school.
Good writeup!
i have a nice laminated one of these in my room! i am taking chemistry next semester @ univeristy! thanks for the information.
ty for the refreshment ;)
I was always interested in chemistry from 7th grade I think, but I never pursued it due to it being pretty damn complicated.
So interesting... I only hope that you can keep this great level of writing in you next posts!
Post a Comment