Inorganic Chemistry studies the formation, composition, structure and chemical reactions of inorganic elements and compounds (except C-H bond compounds, these are Organic Chemistry's work).
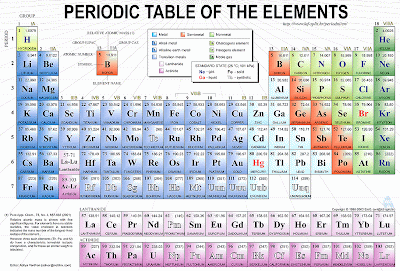
By now you've probably already seen or asked for a PERIODIC TABLE in school, these are called Elements. See how are they arranged?, each element has its specific place on the table thanks to its characteristics, both physical and chemical; like MASS for example, Hydrogen comes to be the lightest Element in the nature, the following elements increase their mass as you go Right or Down.
In the other hand Compounds consists of 2 or more atoms, like Water (H2O).
I will explain more about the periodic table later but for now you should start learning each element’s abbreviation.
Oxygen = O
Hydrogen = H
Helium = He
Calcium = Ca
Easy right?, you will have some problems with elements where the name has nothing to do with the abbreviation but one afternoon repeating them and you will be good to go.
Iron = Fe
Lead = Pb
Mercury = Hg
Just remember, DON’T SKIP THIS STEP, its important for you to recognize each element by its abbreviation or else you will have problems. Don’t go hard on yourself if you can’t memorize them all, I will tell you which are more important (For now) for you to memorize.Concentrate your attention on those elements crossed with the red line, you may leave the rest when your teachers take a test about it.
Each element consists of a single ATOM, in ancient times people believed that the single smallest indivisible particle of the matter was the Atom, now we now it’s not true; atoms consist of 3 particles: Neutrons, Protons and Electrons. There are smaller particles in the atom but this doesn’t concern us…yet.
Many scientists have argued and researched a lot trying to figure out the true structure of the atom, the arrangement of the particles inside of it. Many theories have came out, some pretty crazy, some that made sense.
Atomic Theory
 In 1808, John Dalton tried to explain what was matter made of, stating that all matter consist of little indivisible particles called atoms, and these particles interact with each other forming molecules.
In 1808, John Dalton tried to explain what was matter made of, stating that all matter consist of little indivisible particles called atoms, and these particles interact with each other forming molecules. In 1897, Joseph Thomson took Dalton’s work beyond stating that inside atoms were little particles with Negative charge calling them Electrons, with the nucleus of the atom with a Positive charge.
In 1897, Joseph Thomson took Dalton’s work beyond stating that inside atoms were little particles with Negative charge calling them Electrons, with the nucleus of the atom with a Positive charge.  In 1911, Ernest Rutherford developed a theory closer to the one we use today, he said that atoms have a positive and a negative side, just like Thomson said, but he said that the positive charge was concentrated in the nucleus and electrons orbit around it in circular trajectories. He also predicted the existence of the Neutrons.
In 1911, Ernest Rutherford developed a theory closer to the one we use today, he said that atoms have a positive and a negative side, just like Thomson said, but he said that the positive charge was concentrated in the nucleus and electrons orbit around it in circular trajectories. He also predicted the existence of the Neutrons. In 1913, Niels Bohr proposed an improvement to Rutherford’s theory, saying that electrons didn’t move in 1 single orbit, but in different levels or “distances” of the nucleus, just like the planets around the Sun.
In 1913, Niels Bohr proposed an improvement to Rutherford’s theory, saying that electrons didn’t move in 1 single orbit, but in different levels or “distances” of the nucleus, just like the planets around the Sun.In 1926, Erwin Schrodinger continuing the work of Louis-Victor de Broglie, proposed a new theory, he said that electrons doesn’t behave like particles but like waves, he proposed a mathematical structure leaving behind the “planetary orbits” theory, and introducing a new theory, he said that electrons move around the nucleus but we could only “predict” with mathematical equations where will be more likely to find an electron, giving birth to atomic orbitals, electrons would be in different orbitals depending its energy.
 |
| These are the orbitals where electrons are moving around, following not a single trajectory but a chaotic movement, in the center of these orbitals would be the nucleus |
So basically atoms consist of a NUCLEUS, where NEUTRONS and PROTONS "live", ELECTRONS move around the nucleus in orbitals. Neutrons (with neutral charge) and protons (with positive charge) give the mass to the atom and electrons have negative charge; in Chemical Reactions the elements gain and lose electrons, giving them positive and negative overall charges, these are called IONS.
Coming up: How to use your Periodic Table











8 comments:
This is great!
well the more I know i guess! I never was a science guy so I never took any classes in this area besides the one they make you take in high school.
oh lawd..
Very informative. Could have used this way back when.
i larnd about it is very intresting!
I like this, very interesting
Very educational to say the least!
This topic is very interesting. I usually forget the history of the atomic model and jump straight to the current one
Post a Comment