Petroleum Industry started around 1950, with the first exploration and extraction of this natural resource. The whole Petroleum industry can be described in 3 streams or parts. The Upstream that refers to the exploration and discovery of Petroleum and Gas Sources.
The whole Petroleum industry can be described in 3 streams or parts. The Upstream that refers to the exploration and discovery of Petroleum and Gas Sources.
The Midstream that refers to the small portion of the industry that takes care of the handling, storing and transportation of Petroleum and Gas.
And the Downstream, that is commonly generalized as “Petroleum Industry”, that includes the refining of Crude Oil or Petroleum and Gas.
 The whole Petroleum industry can be described in 3 streams or parts. The Upstream that refers to the exploration and discovery of Petroleum and Gas Sources.
The whole Petroleum industry can be described in 3 streams or parts. The Upstream that refers to the exploration and discovery of Petroleum and Gas Sources. The Midstream that refers to the small portion of the industry that takes care of the handling, storing and transportation of Petroleum and Gas.
And the Downstream, that is commonly generalized as “Petroleum Industry”, that includes the refining of Crude Oil or Petroleum and Gas.
All the Petroleum extracted is transported to a Process Plant where it is refined into other products such as Gasoline, Diesel Oil, Kerosene, Asphalt and others.
 Through the ages we have relied our lives on many energy resources, Petroleum is one of those, mainly for the wide products there can be extracted from it. There are many Oil companies that manage all the Crude Oil market, therefore they control the sub products market aswell.
Through the ages we have relied our lives on many energy resources, Petroleum is one of those, mainly for the wide products there can be extracted from it. There are many Oil companies that manage all the Crude Oil market, therefore they control the sub products market aswell. Many countries have based their economy on the exploration and extraction of Petroleum, spending millions of dollars on it. The technology used on these operations is not cheap, many countries just extract the Crude Oil and then export it to another country with the capabilities to process it. This is when environmental disasters happen, you must have probably heard about Oil disaster on the sea.
It’s all in the carbons, that’s what i always say to explain how Petroleum processing works.
Petroleum is a mixture of several different compounds, all with 1 similar characteristic: They all have Carbons; the more carbons the molecule has, the heavier it is.
Petroleum is a mixture of several different compounds, all with 1 similar characteristic: They all have Carbons; the more carbons the molecule has, the heavier it is.
Compounds on Petroleum have from 1 carbon atom to probably around 20 carbon atoms, this composition depends on the Source of the Petroleum, as in some parts of the world the oil could be heavy or light.
This difference on the weight of the molecules has a direct effect on its boiling point, The boiling point is where the molecule changes from a liquid state to a gas state.
This principle is used on huge distillation columns, this is why if you see an oil industry, they have tall columns.
 These columns are heated on the bottom and as you go higher the temperature decreases, so depending on the height you can extract different compounds of the Oil:
These columns are heated on the bottom and as you go higher the temperature decreases, so depending on the height you can extract different compounds of the Oil: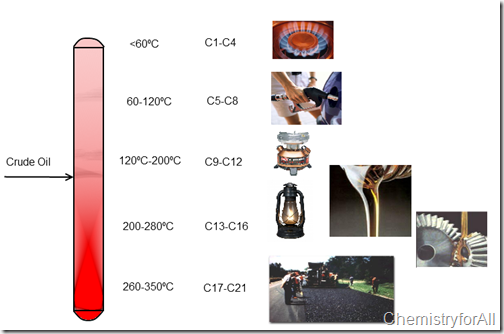 Heavier components such as lubricants, asphalt, kerosene are extracted at the bottom and lighter components like gasoline, propane from the top. This is just a rough example on how Petroleum is refined, it takes several processes and many distillation columns to achieve the final product, also the temperatures and the composition (#of Carbons) are not accurate as some of the final products still have some percentage of some other compounds.
Heavier components such as lubricants, asphalt, kerosene are extracted at the bottom and lighter components like gasoline, propane from the top. This is just a rough example on how Petroleum is refined, it takes several processes and many distillation columns to achieve the final product, also the temperatures and the composition (#of Carbons) are not accurate as some of the final products still have some percentage of some other compounds.Hope you enjoyed it and if you want more information feel free to Contact Us.