Have you ever wondered where Gasoline comes from? You use it everyday on your car but how much do you know about its origin and processing.
 Petroleum, or Oil how it is called in some countries, is a liquid consisting of many organic compounds, these compounds have various molecular structures and were formed beneath the Earth’s surface.
Petroleum, or Oil how it is called in some countries, is a liquid consisting of many organic compounds, these compounds have various molecular structures and were formed beneath the Earth’s surface.Million years ago plants and animals in the oceans died and got trapped under several layers of soil and sand; Time, pressure and Heat turned these rests of animals and plants into oil.
Origin
On Earth there are Carbon-based lifeforms, that includes us, animals and plants; following the Lavoisier Law of Mass conservation, that mass cannot be destroyed but only TRANSFORMED, all the rests of animals and plants trapped under layers and layers of soil and sand suffered changes.
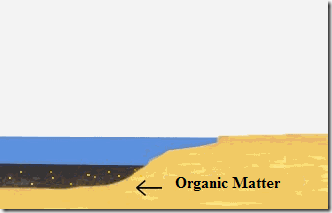
The real origin of oil is uncertain, there are only theories about how it formed.
My favorite is the one that says that tiny animals and plants started to deposit at the bottom of the oceans.
My favorite is the one that says that tiny animals and plants started to deposit at the bottom of the oceans.
 The conditions of pressure and heat, added to the lack of oxygen worked together to promote the formation of liquid petroleum and gas.
The conditions of pressure and heat, added to the lack of oxygen worked together to promote the formation of liquid petroleum and gas.Composition
The composition of Oil is different depending on the source, that is where it was extracted from. Usually it contains around 84% of Carbon and 11% of Hydrogen, the rest are mostly Sulfur and Nitrogen.
There are 4 major groups that are present on Petroleum, depending on their Molecular Structure.
Paraffins
Paraffins are Organic Molecules consisting on C-H linked together by simple bonds, all are lineal and can be branched.
Olefins
Olefins have the same structure than Paraffins, but C-H are linked together by double bonds.
Naphthenes
These organic compounds have C-C and C-H bonds, but the structure have simple bond rings, mostly
Aromatics
Aromatic compounds are similar as Naphthenes, but the only difference is that they have double bond rings, and rings are made out of 6 carbons.
There are other organic compounds that have Nitrogen and Sulfur within their structure.
Fuels and many other products are extracted from this complicated mixture, along with some Gases like methane that are dissolved in the mix.
Fuels and many other products are extracted from this complicated mixture, along with some Gases like methane that are dissolved in the mix.
COMING NEXT!! Petroleum Industry














7 comments:
Man, this just blew my mind - wish I knew more about chemistry, would be awesome. A plan for the holidays!
Interesting :) Though I kinda already knew this.
This is a really great in-depth blog.. I only understand about 50% of it, but it's really good stuff!!
Super informative post. Great read!
Definitely useful to know, so that we can make informed decisions when voting on oil-based legislature!
That's a very informative post.
I was just wondering how engines actually process the gas. This helped me a lot :)
Post a Comment