Electron configuration is the arrangement of electrons of an atom or molecule. It concerns the way electrons can be distributed in the orbitals of the atom.
Orbitals
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom.
It’s a function that can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus.
We will use this term to refer to the physical region defined by the function where the electron is likely to be.
There are 4 kinds of orbitals (recent studies may find new elements that will have 2 extra orbitals):
Orbital “s”
This can be explained with a sphere shape, electrons on this orbital spend their time in some point of this sphere, most likely near the center where the nucleus is, we can see that in this dot representation.
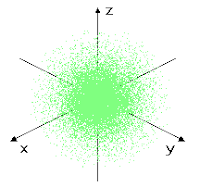 |
| There is only 1 orbital type s |
Orbital “p”
This orbital can be represented by 2 spheres that are lightly shrinked into a connecting point (the nucleus).
 |
| There are 3 orbitals type p |
Orbital “d”
This orbital has diverse shapes, its like 4 lobes joined together in a center that is the nucleus.
 |
| There are 5 orbitals type d |
Orbital “f”
f orbitals have even more exotic shapes that wont be easy to explain but to show:
 |
| There are 7 orbitals type f |
The place of the atom in the periodic table will indicate which orbital is the last orbital of the last shell
Quantum numbers
Because of the quantum mechanical nature of the electrons around a nucleus, they cannot be described by a location and momentum. Instead, they are described by a set of quantum numbers that encompasses both the particle-like nature and the wave-like nature of the electrons.
Every atomic orbital is identified by the values of 3 quantum numbers, the forth number is for the electrons in those orbitals.
The principal quantum number, n, describes the energy of the electron, they are sometimes called electron shells.
Here your Periodic Table comes handy:
This numbers (Periods) tells the number of electron shells the atom has.
The azimuthal quantum number, , describes the orbital angular momentum of each electron. The set of orbitals associated with a particular value of “
, describes the orbital angular momentum of each electron. The set of orbitals associated with a particular value of “ ” are sometimes collectively called a subshell.
” are sometimes collectively called a subshell.
 , describes the orbital angular momentum of each electron. The set of orbitals associated with a particular value of “
, describes the orbital angular momentum of each electron. The set of orbitals associated with a particular value of “ ” are sometimes collectively called a subshell.
” are sometimes collectively called a subshell.
The max number of subshells for a shell is the quantum number minus 1, starting from 0.
n=2 would have 2 quantum numbers ---> 0 and 1
n=4 would have 4 quantum numbers ---> 0, 1, 2 and 3
Electrons on orbital s have  =0
=0
 =0
=0
Electrons on orbital p have  =1
=1
 =1
=1
Electrons on orbital d have  =2
=2
 =2
=2
Electrons on orbital f have  =4
=4
 =4
=4
The magnetic quantum number, m, describes the magnetic moment of an electron in an arbitrary direction.
This quantum number depends on the orbital and the different directions of it. e.g.
Orbital p has 3: px, py and pz, therefore the magnetic quantum number for each p orbital would be -1, 0, 1
This table explains it better:
Orbital
|
m
|
s
|
0
|
p
|
-1 0 1
|
d
|
-2 -1 0 1 2
|
f
|
-3 -2 -1 0 1 2 3
|
Each electron also has a spin quantum number, s, which describes the spin of each electron (spin up or spin down). The number s can be +1⁄2 or -1⁄2.
Number of Electrons
Here your Periodic Table comes into play again, your table gives the information about all the elements in its Ground State, in this state they have neutral charge: Protons (+) are equal to Electrons (-).
The number of protons is given by the Atomic Number: so this is the number of electrons of the element, but in its ground state, not in its ionic (excited) state.
So Oxygen would have 8 electrons
Lead has 82 electrons
Hydrogen has 1
Let’s build electron configurations
First we have to use this chart:
Just follow the line until you run out of electrons, and remember each orbital type can hold up to 2 electrons, so s orbital holds 2 electrons max, p orbital holds 6, d orbital holds 10 and f orbital 14.
Why we do it this way? Because some orbitals must be filled before others, this is because some have more energy than others.
Lets try with some examples:
Carbon, it has 6 for atomic number, so it has 6 electrons aswell.
Using the chart carbon’s electron configuration would be:
C = 1s2 2s2 2p2
Sulfur, has 16 electrons:











5 comments:
Wow, there's a reason why i could never get physics at school and became a lawyer lol
very useful! i remember talking about this in chemestry classes, a long time ago. good to remember! ;)
Real informative, thanks!
That is so way over my head.
Very informative :)
Post a Comment