Every cook in the world is making science, mixing compounds, taking substances to a state where its properties modify.
 |
| Pic Source: http://oodlelinks.com/Cooking.php |
 |
| Pic Source: http://willows95988.typepad.com/tongue_cheek/2008/01/index.html |
 |
| Pic Source: http://lifeslessons-lisa.blogspot.com/2010/11/cooking-sites-and-funny-recipes.html |
 | |
| Pic Source: http://www.destination360.com/australia-south-pacific/australia/sydney/bondi-beach |
 |
| Pic Source: http://www.creighton.edu/ccas/asianworldcenter/publiclecturesandforums/asianethicsforum/index.php |
It is true that the environment would have an effect on how quickly you heat up the water, but this isn't the main factor.
I'm hoping some of you have learned this on school if not i would be very disappointed with your Teachers ^^ , but like i always say it’s never too late to learn new stuff.
Let’s explain first, what does it mean to boil water. The boiling point of ANY substance can be defined like this: “The boiling Point of a substance or element is a temperature where the vapor pressure of the liquid equals the atmospheric pressure in that location”.
Vapor Pressure
What happens when you boil water, It’s nothing geek but take a look for a moment what happens when you start heating water, you see that there is vapor that starts raising up right?, well as the temperature of ANY liquid substance raises this vapor increases.
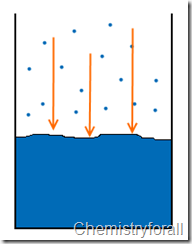 Did you know that a liquid substance constantly evaporates? it is true, and vapor pressure is the pressure of the gas phase over the liquid phase at any given temperature.
Did you know that a liquid substance constantly evaporates? it is true, and vapor pressure is the pressure of the gas phase over the liquid phase at any given temperature.
Atmospheric Pressure
What is Pressure? Pressure is the amount of force per area unit.
The atmospheric pressure can be explained as the amount of Air mass that is above a certain location.
 So the lower your location altitude, the higher is the mass of air above you. and likewise, the higher your location altitude there is less amount of mass over your head therefore the atmospheric Pressure over you is lower than lower altitudes.
So the lower your location altitude, the higher is the mass of air above you. and likewise, the higher your location altitude there is less amount of mass over your head therefore the atmospheric Pressure over you is lower than lower altitudes.This means that as you start heating up water the Vapor pressure increases and HAS to be equal to the atmospheric pressure of your location to hit the boiling point, if the atmospheric pressure is lower than sea level (as a reference sea level atmospheric pressure is around 14.7 psi or 1 Bar or 1 atm., water at this pressure boils at 100ºC or 212ºF) it would take less time to reach that pressure.
- 0 m. (0 ft.) sea level -----> 100ºC (212 ºF) (Reference)
- 2500 m. (8400 ft.) my city -----> around 92ºC (197 ºC) , Here boiling water would take less time than sea level
I'm not taking in consideration that the main purpose of boiling water is to get rid of any kind of microorganism that could have contaminated the water and could give you some sort of disease. In this places it is highly advised even though the water has already boiled to leave it boiling for a few seconds more to be sure all bacteria has been killed.
And as many have noticed already i got a new domain with the help of a friend, hopefully in some time and with some extra money i will get a website hosting service to bring you better content. Stay tuned :)








7 comments:
D; holy crap you opened my eyes, how wrong have i been all this time.. XD... i live at sea level, but water does boil faster in a city we go that is 2200 mts avobe sea level and its always freezing there XD.... +follow
I knew about most of this stuff, but I never actually thought about it when cooking XD I guess you thought me something interesting today, thanks =D
My dad taught me that if you are hiking in the mountains, you need to cook foods in boiling water MUCH longer, hah.
yeah @Randall A., it takes more time to cook foods on the mountains, as the temperature doesnt reach 100ºC and water boils at 75-85ºC, my grandmother also taught me that :) and just now found out the scientific reason hehe
Excellent write up very interesting :)
That's quite interesting.
great post! now all I need is to replace my apron with a lab coat! ;)
Post a Comment